compressed air testing guidelines|Validation of Compressed Air : Pharmag : suppliers In addition, ISO 8573-1 Compressed Air : 2010 also identifies microbiological contaminants. The ISO 8573-1 Compressed Air purity classes can be used to describe the quality of a compressed air system or to specify the required . webAssiste a todos os jogos de futebol online com qualidade HD. MENU; Message to CPRO / DMCA . Está a ver o canal BENFICA TV em stream. Este stream encontra-se na internet. Inácio TV não transmite canais protegidos apenas partilha o que já existe disponível na internet livre. Este site é grátis. Se pagou por este serviço foi enganado!
{plog:ftitle_list}
4 de jan. de 2024 · Las Palmas e Barcelona se enfrentam nesta quinta-feira, às 17h30 (de Brasília), no Gran Canaria, pela 19ª rodada do Campeonato Espanhol. Dez pontos atrás .

When properly treated, compressed air is regarded as a safe, clean utility, as compared to other energy sources. Compressed air provides the energy source for pneumatic conveyers that transport liquids, powders and moisture sensitive product throughout the plant.As a voluntary code of practice, BCAS Food and Beverage Grade Compressed Air .ISO 8573 is an available standard addressing compressed air quality. It .
In addition, ISO 8573-1 Compressed Air : 2010 also identifies microbiological contaminants. The ISO 8573-1 Compressed Air purity classes can be used to describe the quality of a compressed air system or to specify the required .Microbial Testing of Compressed Air Micro Testing of Compressed Air or Bioburden Testing per ISO 8573-7 is generally conducted by the pharmaceutical, medical device and food industries. Microbial contaminants found in the compressor or compressed air lines can be devastating to a final product in these industries. A regular Micro Testing program can provide insight to a .We offer ISO 8573-1 and ISO 8573-7 compressed air testing options. . Trace Analytics can help manufacturers meet custom specifications or international guidelines for gas purity. READ MORE. BREATHING AIR. Compressed .
The FA 510 measures the Pressure dew point down to -80°Ctd. Here too, continuous Measuring ensures that an Alarm can be triggered immediately if the compressed air dryer fails. The sensor enables permanent monitoring of the Compressed air dryer. ISO 8573-4 deals with test methods for the solid particle content.Practical Compressed Air/Gas Testing August 13, 2018 . These guidelines are currently being used as is or modified slightly for specific end uses by hundreds of food, beverage and pharmaceutical processors. The testing methodology is based on the Compressed Gas Association, CGA G-7.1.
oil aerosol in compressed air. This simply alerts you if aerosol is present in your compressed air. A kit is available to order which will allow you (the customer) to take a small air sample and send it to a laboratory for air-testing. Note, this test does not include microorganisms testing. The analysis provided would cover all points in the listISO 8573-1:2010 provides general information about contaminants in compressed-air systems as well as links to the other parts of ISO 8573, either for the measurement of compressed air purity or the specification of compressed-air purity requirements.TRI Air provides equipment to test compressed air samples as part of air quality compliance, safety, SQF. Medical. Solutions for medical gas testing standards including NFPA 99. Fire Service. Compressed breathing air quality for firefighters and SCBA air, NFPA 1989, CGA Grade D, .
Validation of Compressed Air : Pharmag
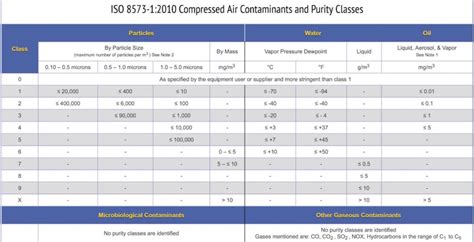
For the test conditions for the water content there are also particular characterstics (maximum 67ppm V/V for compressed gas cylinders or max. 870ppm V/V for compressed air generated by the compressor). The USP (the US Pharmacopoeia) handle the testing of water or oil content quite easily: the so-called mirror test is required. In this piece, we will further explore how each type of impurity can occur and will discuss how to test compressed air/gas quality. Compressed Air Testing Methods. Each of the types of potential air quality issues has different underlying causes and testing methods. In general, compressed air testing is governed by ISO 8573. Depending on the .ISO 8573-2,Compressed air for general use — Part 2: Test methods for aerosol oil content . ISO 8573-3,Compressed air — Part 3: Test methods for measurement of humidity . ISO 8573-4,Compressed air — Part 4: Test methods for solid particle content . ISO 8573-5, Compressed air — Part 5: Determination of oil vapour and organic solvent content.Edition 9 states that a compressed air monitoring plan should test for the following:. Particles: Both viable and non-viable particles concern manufacturers. Improper or inadequate filtration or system contamination can mean these particles may impact the end-products. Water: Excess water in a compressed air system can lead to rust, saturate filters, and create a breeding .
In the absence of specified standards governing compressed air quality testing in the manufacturing process or production of pharmaceutical, medical device, and food/beverage applications, it is often best to use composite, site-specific testing programs. . USP and ISO 8573 air guidelines and standards are common sources from which to draw.Compressed Air Testing Laboratory (512) 263-0498 | [email protected] www.airtesting.com v082516 PRACTICAL ISO 8573-1:2010 COMPRESSED AIR/GAS TESTING GENERAL GUIDELINES AND TESTING ISO 8573-1:2010 SECTION IF APPLICABLE TESTING DESCRIPTION DIRECT PRODUCT CONTACT INDIRECT PRODUCT CONTACT VIABLE .
According to the Compressed Air and Gas Institute (CAGI) and the International Organization for Standardization (ISO), the three major contaminants in compressed air are solid particles, water, and oil. CAGI .
21 CFR 211.46(c) states, in part, that “Air filtration systems, including prefilters and particulate matter air filters, shall be used when appropriate on air supplies to production areas * * *.”%PDF-1.6 %âãÏÓ 17454 0 obj >stream hÞì›[¯$DZ ÿÊ~´ ¤‰[Þ€ :òƒ Ë„(? ‚0–F Š$x Ì ïìÚ½¾&gÆÃáåXÆQ¾ÄÄTDfu¯ŠªZ±;V-{²§Zþä6ö .Start supply of compressed air to the test assembly. 7. If required adjust the flow rate by setting knob of flow meter. 8. Record the time. 9. On completion of 20 minutes stop supply of compressed air to test assembly. 10. Record the reading for oil content shown on the scale by pale blue color. 11. Detach Gastec tube from the test assembly.
Regular testing of compressed air systems and other process gases that come into contact with pharmaceutical products is critical to ensuring the quality and integrity of the product. At Trace Analytics, our team of experts is dedicated to providing safe, clean air at the highest quality standards while simultaneously offering guidance for .Compressed breathing air must meet at least the requirements for Type 1 - Grade D breathing air described in ANSI/CGA G-7.1-1989 . Absent such testing, all other SARs with helmets/hoods are to be treated as loose-ftting facepiece respirators, and receive an APF of 25. (29 CFR 1910.134(d)(3)(i)(A))
At CEGTH, we offer effective testing solutions for your compressed air systems as per the guidelines of ISO: 8573/ USP/EP/BP. Breathing Air Validation. The Compressed Gas Association sets quality standards for breathing air is GRADE D (ANSI/CGA G-7.1’97) CEGTH offers testing of breathing air quality for the following parameters: 5.3 Test for Oil and Moisture Content Note: These tests are applicable for Compressed air points only. 5.3.1 This test shall be performed by the external testing laboratory as per protocol or by using gas detector tubes (Gastec). 5.3.2 Frequency Sample Point from Compressed Air Generation System: Once a Month
Using ISO 8573
PRACTICAL ISO 8573-1:2010 COMPRESSED AIR/GAS TESTING GENERAL GUIDELINES AND TESTING ISO8573-1:2010 SECTION IF APPLICABLE TESTING DESCRIPTION DIRECT PRODUCT CONTACT INDIRECT PRODUCT . compressed air testing kits are developed with detailed awareness of user needs, the location at which samples must be collected, the typesISO8573 - the compressed air quality standard ISO8573 is the group of international standards relating to the quality (or purity) of compressed air. The standard consists of nine separate parts, with part 1 specifying the quality requirements of the compressed air and parts 2 – 9 specifying the methods of testing for a range of contaminants.operations. Compressed -air systems are defined as a group of subsystems composed of air compressors, air treatment equipment, controls, piping, pneumatic tools, pneumatically powered machinery, and process applications using compressed air. A compressed-air system has three primary functional subsystems: supply, distribution, and demand.Additional fittings shall be provided so that test gauges may be attached whenever necessary. 1926.803(g)(1)(vi) . In addition to fire hose protection required by this subpart, on every floor of every building not under compressed air, but used in connection with the compressed air work, there shall be provided at least one approved fire .
Testing and monitoring compressed air and other process gases, such as gaseous and liquid nitrogen, oxygen, argon, and carbon dioxide, that come into direct contact with pharmaceutical drugs during the manufacturing process is vital to assuring the quality and safety of these products.
v50 ballistic impact test
vaccine impact antigen test
webfevereiro 2024. sex 01. março 2024. sáb 02. março 2024. dom 03. março 2024. seg 04. março 2024. 05h19. |. RELATÓRIO AR BTV. REP. RELATÓRIO AR BTV 1/1. +. 05h43. |. .
compressed air testing guidelines|Validation of Compressed Air : Pharmag